Since
the COVID-19 outbreak emerged in the U.S., federal guidance and state orders
have been released in waves over the last several months having an especially
drastic effect on healthcare that extends beyond the care of patients infected
with the virus. Many states have now mandated the suspension of elective
medical services. When response efforts are locally executed and state managed,
however, disparities emerge over what governments consider to be “elective”. As
a result, while many healthcare services have remained uninterrupted, surgical
abortions are being called into question as to whether they should be
considered “elective,” and therefore suspended during this time.
What
is an essential business?
On
March 16, 2020, the President and the Coronavirus Task Force recommended that
civilians work from home while calling for those who work in critical
infrastructure industries to remain in operation. The “Essential Critical
Infrastructure Workforce” advisory list was developed to help state officials
determine which businesses and services should remain operational during this
period.
State
interpretations
To
create capacity and meet the increases in resource demands, many states have
chosen to follow CDC guidelines stating that “healthcare
facilities and clinicians should prioritize urgent and emergency visits and
procedures…”
In
states where surgical abortion procedures were previously facing challenges,
the term “elective” in these materials has given states significant latitude in
determining what patients need, under what terms, and when. The argument for
restricting elective or non-urgent medical procedures is not without merit. The
U.S. is experiencing a shortage of personal protective equipment and medical
supplies leaving states to ration existing provisions. Most states have required
providers to suspend non-urgent services such as annual physicals, dental
check-ups, cosmetic procedures, and routine screenings such as colonoscopies
and mammograms.
Although a patient may choose to receive an abortion, relying on this choice to classify surgical abortions as elective results in unique issues unfaced by other elective-designated medical procedures. Abortions are time sensitive. Most acutely in states that have reduced the window of time a patient may obtain an abortion, requiring a patient to wait until after the outbreak jeopardizes the opportunity they have to access this service. This is especially challenging when states are consistently moving back the date upon which elective or nonessential medical services can be resumed as new information emerges on the severity of state outbreaks. Historically, we know that restricting access to surgical abortions does not decrease the need for their services. Women who are unable to obtain an abortion will either require complex surgical procedures for later-term abortions, remain pregnant and require prenatal care and delivery services, or may use dangerous methods to induce an abortion on their own (UCSF Bixby Center). The side effects of suspending surgical abortions would result in more frequent clinical visits (i.e. prenatal care) or longer admissions (i.e. later-term abortions, self-induction, delivery) in the hardest hit clinical settings these restrictions are trying to protect. If suspending elective medical procedures is also a tactic to reduce social contact among patients and with medical providers, restricting abortions will likely result in women traveling to other states where the service is preserved, increasing the chance for viral mobility and exposure.
Below
is a summary of the states that have classified abortions as “elective” during
the pandemic and states that are facing challenges to these determinations in
court.
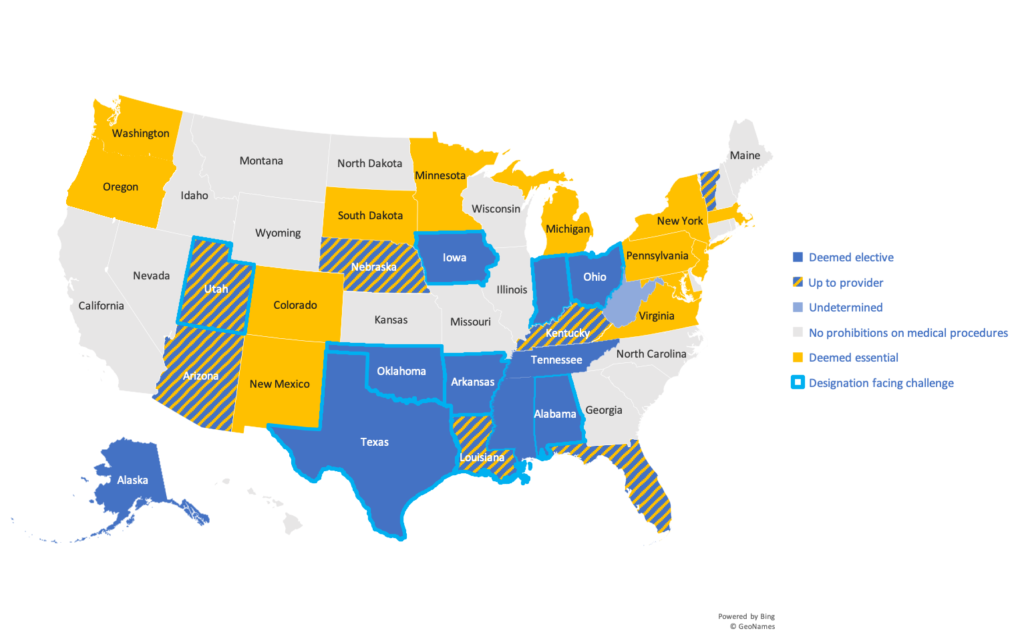
Additional
information on state-specific mandates/guidance can be found here. Challenges to these
designations in court have been summarized here.
Post-pandemic
The
COVID-19 outbreak has illuminated many vulnerabilities in our healthcare
system. Prior to the pandemic, abortion services were facing renewed challenges
in courts. Classifying abortions as “elective”, however, perpetuates the
rhetoric that abortions are chosen luxuries when women often face little choice
in the nonmedical reasons they have to obtain an abortion. It is difficult to
see through the thicket of disparate recommendations and orders made by state
and local governments, but it is clear that the end of the pandemic will not
eliminate the challenges raised by these regulations nor the discretion states
may take in the future in deeming what is and is not essential medical care.
